38 fda requirements food labels
CFR - Code of Federal Regulations Title 21 - Food and Drug ... Sec. 101.9 Nutrition labeling of food. (a) Nutrition information relating to food shall be provided for all products intended for human consumption and offered for sale unless an exemption is... How To Read Food and Beverage Labels | National Institute ... How to read the Nutrition Facts label. The U.S. Food and Drug Administration (FDA) requires a Nutrition Facts label on most packaged foods and beverages. At the top of the Nutrition Facts label, you will find the total number of servings in the container and the food or beverage's serving size.
Code of Federal Regulations Title 21 - Food and Drug ... (a) (1) ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be...
Fda requirements food labels
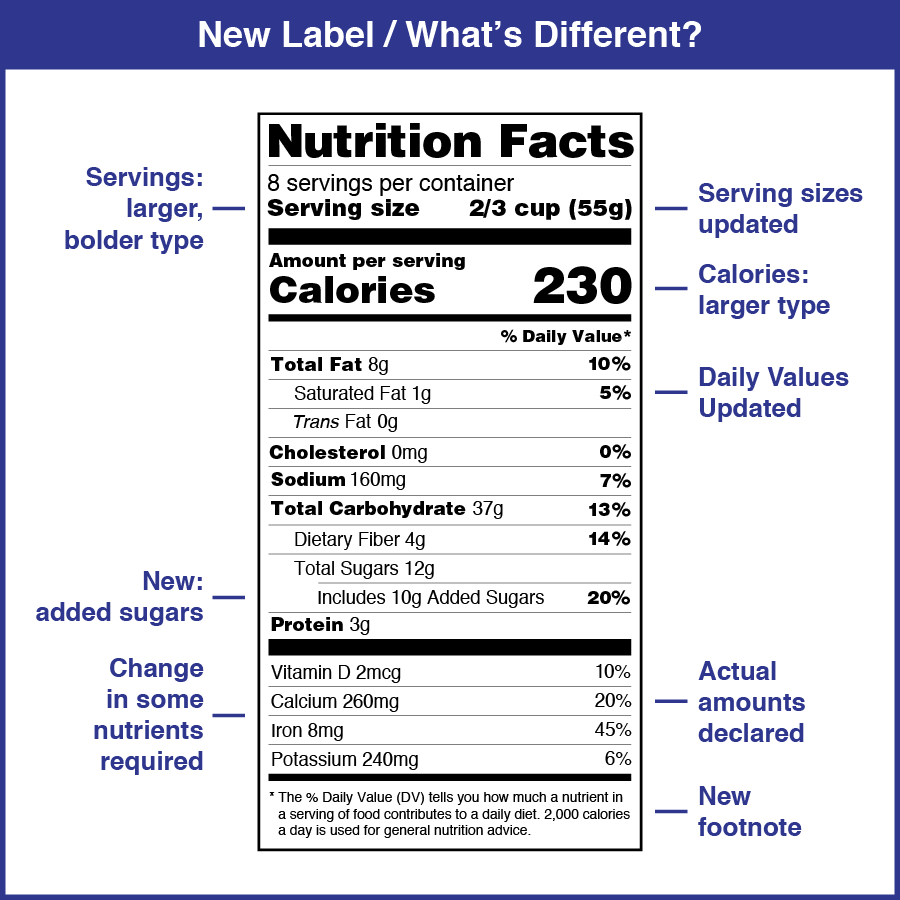
FSIS Labeling Overview and Generic Label Approval | Food ... This guidance also provides additional instruction on required labeling features, new generic labeling regulations, sample labels, label submission, and labeling records. It applies to food manufacturers and retailers and the actions they may take to comply with the label requirements applicable to meat and poultry. It relates to 9 CFR 412. Learn How the Nutrition Facts Label Can Help You Improve ... The label is required on all packaged foods made in the United States and imported from other countries. The US Food and Drug Administration (FDA) issued regulations in 2016 to update the Nutrition Facts label. This was the first major change to the label since it was introduced in 1994. Most items had the updated label by January 1, 2021. FDA Food Product Labeling & Packaging Requirements | ESHA ... Mandatory nutrients (total calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrate, dietary fiber, total sugars, added sugars, protein, vitamin D, calcium, iron, potassium) Placement: In general, place the Nutrition Facts Label on the PDP or the Information Panel, near the ingredient statement. Ingredient Statement
Fda requirements food labels. Guidance for Industry: Food Labeling Guide | FDA Under FDA's laws and regulations, FDA does not pre-approve labels for food products. Questions concerning the labeling of food products may be directed to the Food Labeling and Standards Staff ... Food Labeling Modernization Act of 2021: What the Bill ... The Food Labeling Modernization Act (FLMA) of 2021, an update of legislation originally introduced to Congress in 2018, was presented to the House and Senate in August.As written, the bill would require FDA to establish a standard front-of-package labeling system for all FDA-regulated food products. FDA Food Packaging Guidelines for 2022 | Newprint Nutritional labels must be printed in all black or one colour type on a white or neutral contrasting background and must be readable. There have been changes in the FDA food labeling requirements regarding nutrition facts, and the transition period has ended on January 1, 2021. Health Canada closely regulates the size and appearance of the NFT. Code of Federal Regulations Title 21 - Food and Drug ... Requirements of conspicuousness and legibility shall include the specifications that: (1) The ratio of height to width (of the letter) shall not exceed a differential of 3 units to 1 unit (no more...
Food Labeling & Nutrition | FDA 25/03/2022 · What's new in food labeling and nutrition, including label claims, nutrition labeling for restaurants, and links to industry guidance. What are the Requirements for a Food Label? - Short Food ... Required Food Label Information The FDA requires seven areas of information on food labels for legal sale of these goods. These items include the following information about the food product. All labeling must be in English, though some foreign language is appropriate so long as the English translation is also present Code of Federal Regulations Title 21 - Food and Drug ... (a) You must take necessary actions to determine whether packaging for dietary supplements meets specifications so that the condition of the packaging will ensure the quality of your dietary... Label Claims for Conventional Foods and Dietary ... there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990...
How to Make Food & Beverage Labels - Avery An important part of a food and beverage label is the nutritional information.The FDA has updated the Nutrition Facts label on packaged foods and drinks and is requiring changes based on updated scientific information, new nutrition research, and input from the public. This is the first major update to the nutrition label in more than 20 years. The New Nutrition Facts Label | FDA The U.S. Food and Drug Administration (FDA) has updated the Nutrition Facts label on packaged foods and drinks. FDA is requiring changes to the Nutrition Facts label based on updated scientific... USA Food Labeling Regulations - FDABasics Food product labeling requirements in the USA for Animal food, Human Food, and Human Dietary supplements are different. FDA does not approve the food product label. However, failure to comply with food labeling requirements may result in enforcement action such as detention, warning letter, refusal to entry, or import alert. New FDA Food Labeling Guidelines Include Nutrition Facts ... US Food and Drug Administration FDA Food Labeling Guidelines. The US FDA announced new FDA food labeling guidelines for food packaging. The new labels would reflect scientific information, "including the link between diet and chronic diseases such as obesity and heart disease". The new FDA food labeling is being done to make it easier for ...
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and...
CFR - Code of Federal Regulations Title 21 - Food and Drug ... Subpart B - Specific Food Labeling Requirements § 101.22 - Foods; labeling of spices, flavorings, colorings and chemical preservatives. § 101.30 - Percentage juice declaration for foods purporting...
Food Allergies | FDA The FDA requires all products containing FD&C Yellow No. 5 to identify it on their labels so consumers who are sensitive to the dye can avoid it. Color additives made from cochineal extract and...

U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements - Viva FDA - U.S. FDA ...
CFR - Code of Federal Regulations Title 21 - Food and Drug ... (3) section 403 (i) (1) of the act (requiring the label to bear the common or usual name of the food), if the food is displayed to the purchaser with its interstate labeling clearly in view, or...
Code of Federal Regulations Title 21 - Food and Drug ... (h) The label of a food to which flavor is added shall declare the flavor in the statement of ingredients in the following way: (1) Spice, natural flavor, and artificial flavor may be declared as...
What are the FDA Labeling Requirements for Cosmetic Products? This Act requires that all consumer commodities should be labeled in a way that reveals the amount of product in the package, the identity of the commodity, as well as the name/place of business of the product's manufacturer. The Act also allows for additional regulations to prevent other forms of consumer deception due to labeling.
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
Changes to the Nutrition Facts Label | FDA The updated label appears on the majority of food packages. Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less...
Organic on Food Labels | FDA Food products that are ordinarily under FDA's jurisdiction and labeled with organic claims must comply with both USDA NOP regulations for the organic claim and FDA regulations for food labeling and...
Create a Nutrition Label - LabelCalc - FDA Compliant Label Formats. Last but certainly not least, to create a nutrition label that is FDA-compliant, you must choose the correct label format based on your product package size. Within the LabelCalc platform, you can select a format to either update your labels to 2020 format or create labels in the latest 2020 FDA-Required format for food products.
FDA Labeling Regulations | UC Food Safety Food Labeling & Nutrition (FDA) Nutritional labels are required on most food products. Small businesses can claim an exemption from Nutritional Labeling requirements. However, it is common to have a nutritional label prepared and available for customers upon request even if it doesn't appear on the label.
FDA Updates on Paxlovid for Health Care Providers | FDA FDA Updates on Paxlovid for Health Care Providers. FDA authorized Paxlovid (nirmatrelvir and ritonavir) in December 2021 for the treatment of mild-to-moderate COVID-19 in adults and pediatric ...
FDA Food Product Labeling & Packaging Requirements | ESHA ... Mandatory nutrients (total calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrate, dietary fiber, total sugars, added sugars, protein, vitamin D, calcium, iron, potassium) Placement: In general, place the Nutrition Facts Label on the PDP or the Information Panel, near the ingredient statement. Ingredient Statement
Learn How the Nutrition Facts Label Can Help You Improve ... The label is required on all packaged foods made in the United States and imported from other countries. The US Food and Drug Administration (FDA) issued regulations in 2016 to update the Nutrition Facts label. This was the first major change to the label since it was introduced in 1994. Most items had the updated label by January 1, 2021.
FSIS Labeling Overview and Generic Label Approval | Food ... This guidance also provides additional instruction on required labeling features, new generic labeling regulations, sample labels, label submission, and labeling records. It applies to food manufacturers and retailers and the actions they may take to comply with the label requirements applicable to meat and poultry. It relates to 9 CFR 412.









:max_bytes(150000):strip_icc()/__opt__aboutcom__coeus__resources__content_migration__treehugger__images__2014__02__new_vs_old_nutrition_facts_label-aaed1f9fbeee44de91b5bba54f14f9ef.jpg)
Post a Comment for "38 fda requirements food labels"