41 medication labels must include
FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,... Ch 8 Pharmacy Flashcards | Quizlet A legal prescription label must include all of the following except: A. Directions for use B. Date the prescription was dispensed C. Name, address, and telephone number of the prescriber D. Name, address, and telephone number of the dispensing pharmacy Name, address, and telephone number of the prescriber
How to Label Prescription Medication for Veterinary Patients A label should include the following components: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's...

Medication labels must include
Labeling Information | Drug Products | FDA For more information on labeling, including Physician Labeling Rule (PLR) requirements, guidances, presentations, sample templates and format tools, and established pharmacologic class (EPC ... Guidance Document: Labelling of Pharmaceutical Drugs for Human Use (1.4) When the composition of the drug varies from one lot to another, the outer label must include a reference to all non-medicinal ingredient alternatives that may be present in the drug, ... Claims on drug product labels that include market share, sale, consumer and patient use/ choice, or preference must be supported by adequate studies ... Medication Administration Safety - Patient Safety and Quality Threats to medication safety include miscommunication among health care providers, drug information that is not accessible or up to date, confusing directions, poor technique, inadequate patient information, lack of drug knowledge, incomplete patient medication history, lack of redundant safety checks, lack of evidence-based protocols, and staff assuming roles for which …
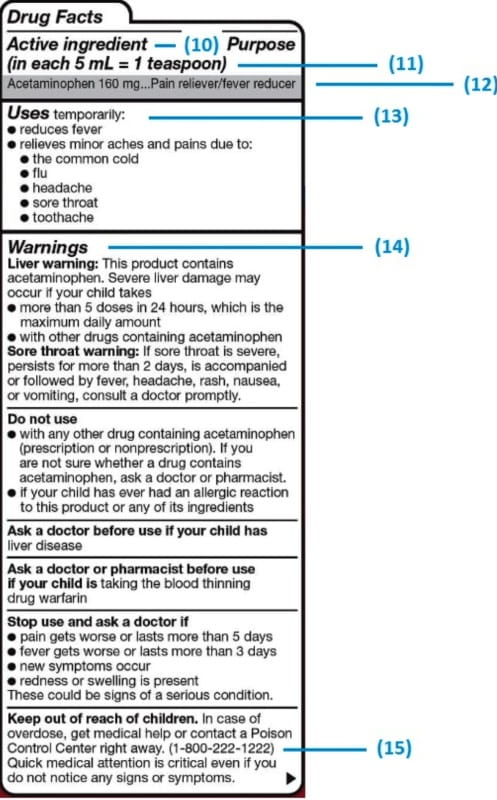
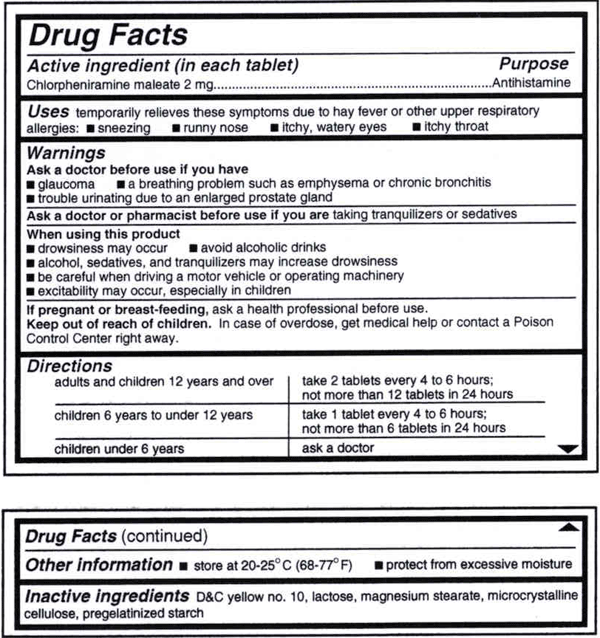
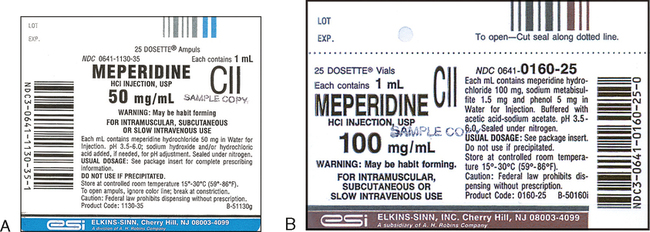
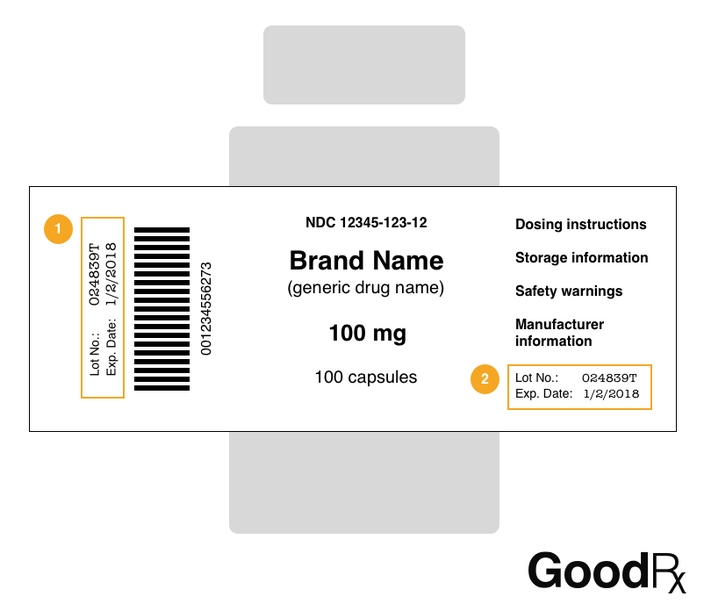
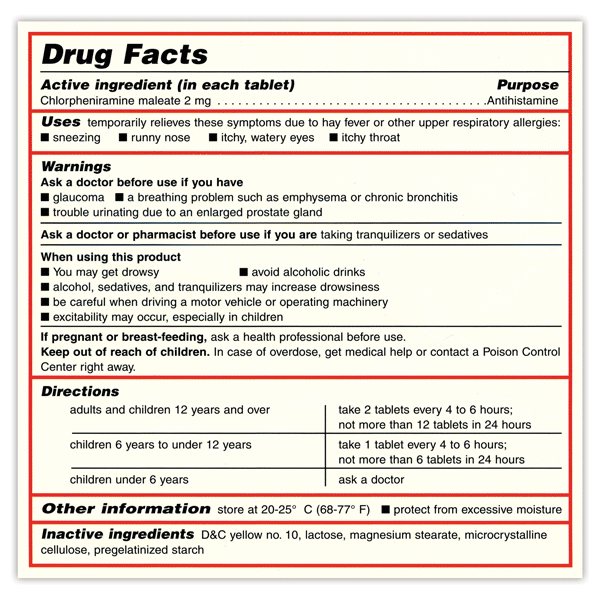
Medication labels must include. Lexis | Online Legal Research | LexisNexis The surprising truth about content … Fact: Lexis ® has the largest collection of case law, statutes and regulations.* Plus 40K+ news sources, 83B+ Public Records, 700M+ company profiles and documents, and an extensive list of exclusives across all … Drug labeling, Information about Drug labeling - FAQs Each product must contain a label with "Supplement Facts" in bold letters onthe front panel. This is the manufacturer's opportunity to identify the product. Below "Supplement Facts," the panel must state the serving size. This isdetermined by the manufacturer with no input from the FDA. Pharmaceutical Labeling: Requirements & Guidelines - CTM Labeling Systems To meet today's FDA regulations, labeling information on drugs must include the following in this order: - Product Name - Drug Facts Table - Active Ingredients - Purpose and Use - Warnings - Directions - Allergic Reactions - Inactive Ingredients FDA Issues New RX Label Requirements - The Recovery Village Drug and ... A human prescription drug label must include all of the legal information set forth by the FDA. Details on Abuse-Deterrent: The information that must be clearly printed on containers of prescription drugs is meant to immediately identify the origin and recipient of the drug, including where and by whom it was prescribed and consumption ...
abcnews.go.com › healthHealth News | Latest Medical, Nutrition, Fitness News - ABC ... Get the latest health news, diet & fitness information, medical research, health care trends and health issues that affect you and your family on ABCNews.com FDA Says Drug Labels Must Include Clear Guidance for ... - Healthline Starting June 30, new drug labels will have categories for "Pregnancy," "Lactation," and "Females and Males of Reproductive Potential." "Pregnancy" will include information on ... › books › NBK2656Chapter 37 Medication Administration Safety - NCBI Bookshelf Threats to medication safety include miscommunication among health care providers, drug information that is not accessible or up to date, confusing directions, poor technique, inadequate patient information, lack of drug knowledge, incomplete patient medication history, lack of redundant safety checks, lack of evidence-based protocols, and staff assuming roles for which they are not prepared. › TR › html4Forms in HTML documents - W3 In an HTML document, an element must receive focus from the user in order to become active and perform its tasks. For example, users must activate a link specified by the A element in order to follow the specified link. Similarly, users must give a TEXTAREA focus in order to enter text into it. There are several ways to give focus to an element:
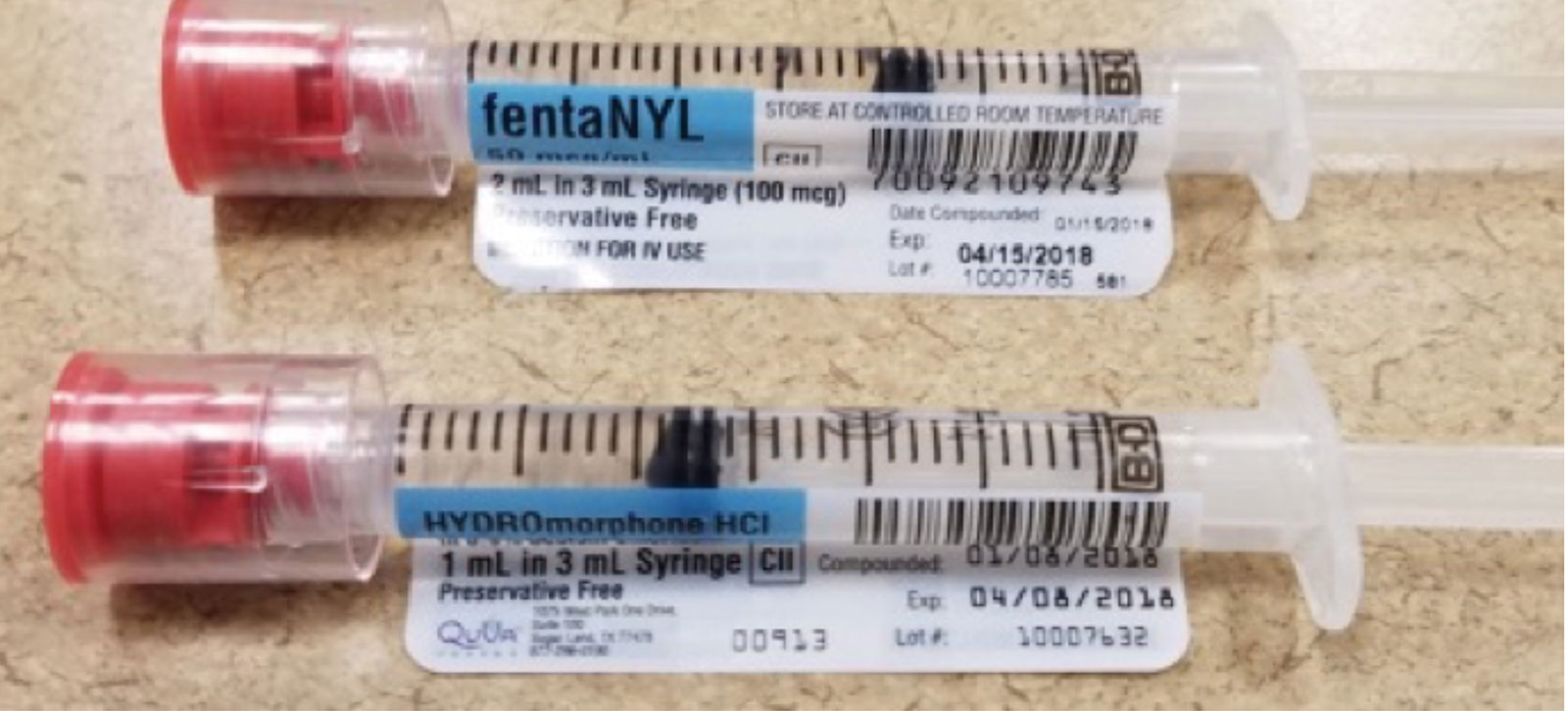
Your Guide to Medication Labeling - Outpatient Surgery Magazine ... Here's a guide to help you make sure that medications are properly labeled in your facility. Any drawn medication the provider sets down on or off the sterile field should be properly labeled. Syringes require 5 labeling elements: drug, strength, date, time drawn and the initials of the person who prepared the syringe. Carbohydrates and Blood Sugar - The Nutrition Source When people eat a food containing carbohydrates, the digestive system breaks down the digestible ones into sugar, which enters the blood. As blood sugar levels rise, the pancreas produces insulin, a hormone that prompts cells to absorb blood sugar for energy or storage. RXinsider | How to Label a Medication Syringe At a minimum, medication containers and medication syringe labels must include: • Accurate spelling of medication name • Brand name or generic name • Patient's name • Dosing amounts • Dosing and/or drug administration instructions • Total medication quantity • Medication expiration date • Date of dispensing • Serial number • Name of the prescriber Best practice in the labelling and packaging of medicines Guidance Best practice in the labelling and packaging of medicines This guidance explains the legal framework for labelling and packaging as described in UK legislation and gives best practice for...
NIMH » Mental Health Medications The FDA requires that all antipsychotic medication labels include a black-box warning stating that antipsychotics are associated with increased rates of stroke and death in older adults with dementia. ... People who take clozapine must have regular blood tests to check for a potentially dangerous side effect that occurs in 1% to 2% of people.
en.wikipedia.org › wiki › Medication_package_insertMedication package insert - Wikipedia In the United States, the Food and Drug Administration (FDA) determines the requirements for patient package inserts. In the United States, the FDA will occasionally issue revisions to previously approved package inserts, in much the same way as an auto manufacturer will issue recalls upon discovering a problem with a certain car.
Health News | Latest Medical, Nutrition, Fitness News - ABC News - ABC News 10/8/2022 · Get the latest health news, diet & fitness information, medical research, health care trends and health issues that affect you and your family on ABCNews.com
› mental-health-medicationsNIMH » Mental Health Medications Antipsychotic treatment for older adults necessitates additional care and consideration. The FDA requires that all antipsychotic medication labels include a black-box warning stating that antipsychotics are associated with increased rates of stroke and death in older adults with dementia.
Safe Labeling Helps Prevent OR Medication Errors - OR Today Label information must include a medication's name and strength as well as amount when medications are mixed (as with antibiotic irrigations, tumescent and heparin solutions, and epinephrine). The unit of measure — percent, grams, milliliters, or units — must be recorded along with the date the medication is prepared.
About List N: Disinfectants for Coronavirus (COVID-19) | US EPA 5/24/2022 · EPA expects all products on List N to kill the coronavirus SARS-CoV-2 (COVID-19) when used according to the label directions.
Medicines: packaging, labelling and patient information leaflets Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional warning statements must be included on the packaging of...
A Safer Way to Administer Medication Starts with Improving ... The five "rights" method & understanding medication bag labeling. Labeling medication bags is a key part of the "five rights" method for safe medication practices. This entails administering the right medication, in the right dose, at the right time, by the right route, and to the right patient 1. Line or drug infusion bag labels help ...
The Over-the-Counter Medicine Label: Take a Look | FDA All nonprescription, over-the-counter (OTC) medicine labels have detailed usage and warning information so consumers can properly choose and use the products. Below is an example of what the OTC...
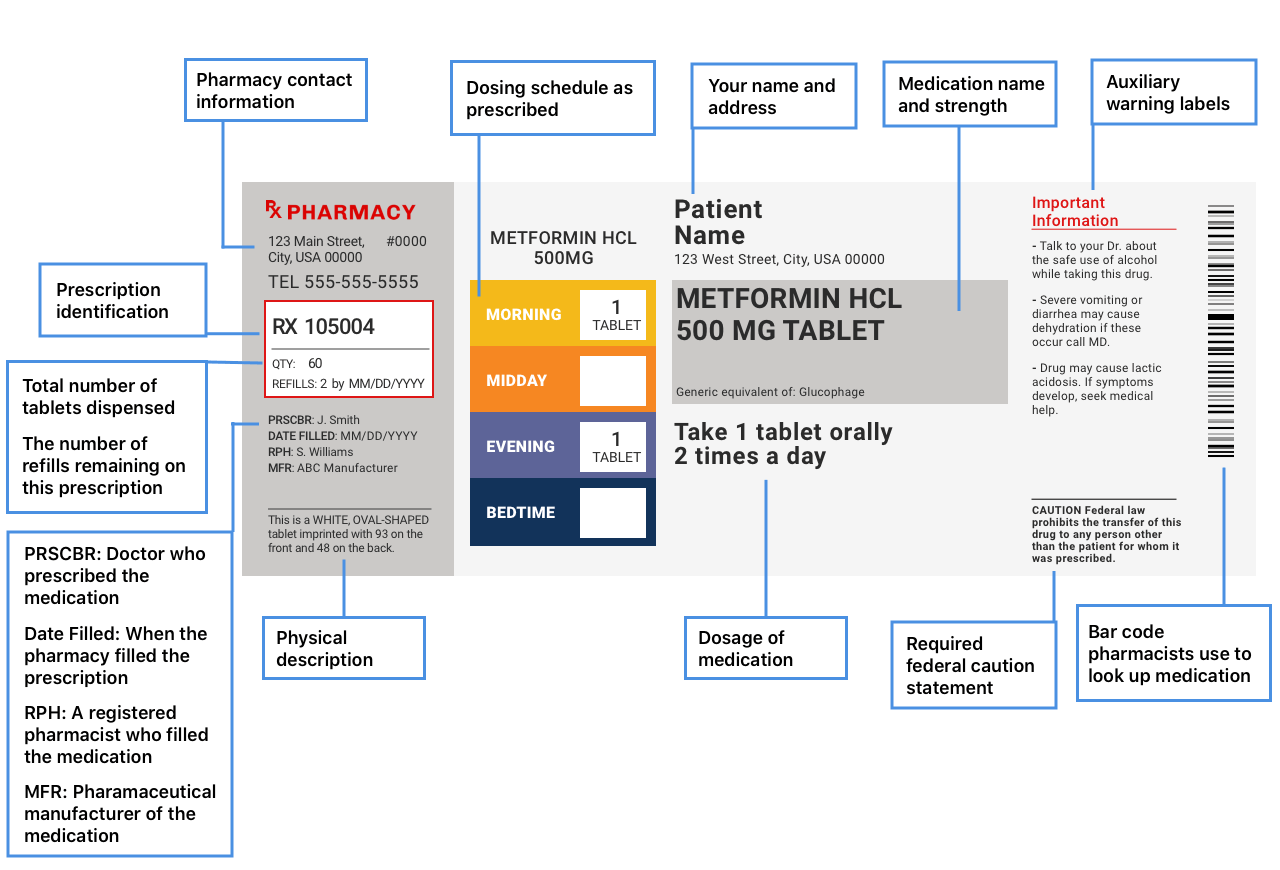
Chapter 5: Prescriptions and Labels Flashcards | Quizlet Drug Labels Regulated by the Food and Drug Administration (FDA), which determines what needs to be on the label Dispensing pharmacist's label must include: Pharmacy name, address, and phone number Dispensing date Dispensing date may differ from the date on the prescription. Rx number, which identifies this unique prescription in the computer system
Forms in HTML documents - W3 17.1 Introduction to forms. An HTML form is a section of a document containing normal content, markup, special elements called controls (checkboxes, radio buttons, menus, etc.), and labels on those controls. Users generally "complete" a form by modifying its controls (entering text, selecting menu items, etc.), before submitting the form to an agent for processing (e.g., to a Web server, …
› coronavirus › about-list-nAbout List N: Disinfectants for Coronavirus (COVID-19) | US EPA May 24, 2022 · EPA expects all products on List N to kill the coronavirus SARS-CoV-2 (COVID-19) when used according to the label directions.
Hypoglycemia (Low Blood Glucose) | ADA - American Diabetes … Each person's reaction to low blood glucose is different. Learn your own signs and symptoms of when your blood glucose is low. Taking time to write these symptoms down may help you learn your own symptoms of when your blood glucose is low. From milder, more common indicators to most severe, signs and symptoms of low blood glucose include ...
Pharmaceutical Labeling 101: FDA Regulations Guide These include drugs like analgesics, anti-inflammatory agents, antibacterial, anticonvulsants, and others. The substance is used in the diagnosis, mitigation, cure, treatment, or prevention of diseases. This category also includes supplements. The substance is a component of medication but not a part of a medical device.
How to Label a Medication Syringe - Medical Packaging Inc., LLC At a minimum, medication containers and medication syringe labels must include: Accurate spelling of medication name Brand name or generic name Patient's name Dosing amounts Dosing and/or drug administration instructions Total medication quantity Medication expiration date Date of dispensing Serial number Name of the prescriber
What Information Should Be on Drug Labels? - MedicineNet Certain information must be included on a prescription drug label. The FDA requires prescription labeling to be printed with: Pharmacy information The doctor's information Instructions Physical description of the drug Federal caution statement Dates Pharmacy prescription number Number of pills Number of times the drug can be reordered
Medication package insert - Wikipedia A package insert is a document included in the package of a medication that provides information about that drug and its use. For prescription medications, the insert is technical, providing information for medical professionals about how to prescribe the drug. Package inserts for prescription drugs often include a separate document called a "patient package insert" with …
› government › publicationsSEND code of practice: 0 to 25 years - GOV.UK Jun 11, 2014 · 30 April 2020. Added link to guidance on 'Changes to the law on education, health and care needs assessments and plans due to coronavirus'. 1 May 2015
SEND code of practice: 0 to 25 years - GOV.UK 6/11/2014 · 30 April 2020. Added link to guidance on 'Changes to the law on education, health and care needs assessments and plans due to coronavirus'. 1 May 2015
Medication Administration Safety - Patient Safety and Quality Threats to medication safety include miscommunication among health care providers, drug information that is not accessible or up to date, confusing directions, poor technique, inadequate patient information, lack of drug knowledge, incomplete patient medication history, lack of redundant safety checks, lack of evidence-based protocols, and staff assuming roles for which …
Guidance Document: Labelling of Pharmaceutical Drugs for Human Use (1.4) When the composition of the drug varies from one lot to another, the outer label must include a reference to all non-medicinal ingredient alternatives that may be present in the drug, ... Claims on drug product labels that include market share, sale, consumer and patient use/ choice, or preference must be supported by adequate studies ...
Labeling Information | Drug Products | FDA For more information on labeling, including Physician Labeling Rule (PLR) requirements, guidances, presentations, sample templates and format tools, and established pharmacologic class (EPC ...























Pharmaceutical quality assurance companies often work closely with pharmaceutical manufacturers to implement best practices in labeling. This involves staying abreast of industry developments, incorporating the latest technologies, and ensuring that labeling processes align with the highest standards.
ReplyDelete